What Are Mitochondria?
Mitochondria are tiny organelles found in almost every cell in the human body, often referred to as the “powerhouses” of the cell. These small structures are responsible for generating the energy that cells need to function. On average, each cell contains about 100 mitochondria, though the number can vary depending on the cell’s energy requirements.
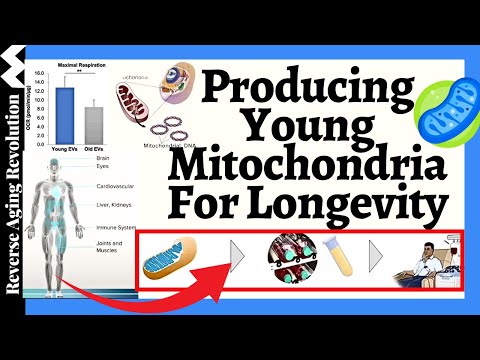
The Role of Mitochondria in Energy Production
Mitochondria are critical for cellular energy production. They accomplish this by converting glucose and oxygen into adenosine triphosphate (ATP), the energy currency of the cell. This process, known as cellular respiration, is vital for all cellular activities, from muscle contraction to brain function. Without efficient ATP production, cells cannot perform their necessary functions, leading to various health issues.
The Unique Structure of Mitochondrial DNA
Mitochondria contain their own genetic material, known as mitochondrial DNA (mtDNA), which is distinct from the nuclear DNA found in the cell’s nucleus. Unlike nuclear DNA, which consists of approximately three billion base pairs, mtDNA is much smaller, comprising only about 16,000 base pairs. This circular DNA is critical for encoding the proteins required for mitochondrial function.
The Evolutionary Origins of Mitochondria
Mitochondria as Ancient Bacteria
The theory of endosymbiosis suggests that mitochondria originated from ancient bacteria that were engulfed by a primitive eukaryotic cell over a billion years ago. This symbiotic relationship allowed the host cell to harness the bacteria’s energy-producing capabilities, leading to the evolution of complex multicellular organisms, including humans. The similarities between mitochondria and bacteria, such as their circular DNA and double membrane structure, support this theory.
The Inheritance of Mitochondrial DNA
Mitochondrial DNA is inherited maternally, meaning it is passed down from mother to child with no contribution from the father. This unique inheritance pattern allows for the direct transmission of mtDNA across generations, making it a valuable tool for tracing lineage and studying evolutionary history.
The Decline of Mitochondrial Function with Age
Mitochondrial Decline and Aging
As we age, the function of our mitochondria declines, leading to a decrease in cellular energy production. This decline is one of the underlying causes of many age-related diseases. At the age of 20, when mitochondrial function is at its peak, cells are energized, and the body operates at optimal efficiency. However, as we grow older, the number and efficiency of mitochondria decrease, contributing to the onset of diseases such as cardiovascular disease, Alzheimer’s, diabetes, and arthritis.
The Impact of Mitochondrial Decline on Disease
Mitochondrial decline doesn’t just affect general health; it also plays a significant role in the development and progression of various diseases. When mitochondria are unable to produce sufficient ATP, cells cannot perform their functions effectively, leading to tissue damage and the progression of diseases. For example, in Alzheimer’s disease, reduced mitochondrial function may lead to neuronal damage and cognitive decline. Similarly, in cardiovascular diseases, impaired energy production can weaken heart muscles, leading to heart failure.
Causes of Mitochondrial Decline
Mitochondrial decline can be accelerated by various factors, including lifestyle choices such as smoking, excessive alcohol consumption, and poor diet. Additionally, chronic diseases like multiple sclerosis can cause the body to continuously battle stress, leading to faster mitochondrial degradation. This accelerated decline contributes to premature aging and an increased risk of age-related diseases.
Investigating Mitochondrial DNA Damage
Analyzing Mitochondrial DNA Damage
Recent studies have enabled scientists to analyze mitochondrial DNA damage as a way to measure aging. By sequencing the mtDNA, researchers can identify errors or mutations that accumulate over time. For instance, in a study where a 94-year-old woman’s mtDNA was sequenced, it was found that she had a 25% damage score, indicating a significant amount of accumulated damage. This damage was compared to younger individuals, such as her son with an 11% damage score and her grandson with a 7% damage score, showing a clear decline in mitochondrial health with age.
The Role of Mitochondrial DNA in Aging
Mitochondrial DNA is constantly replicating, and with each replication, there is a chance for errors to occur. These errors accumulate over time, leading to a gradual decline in mitochondrial function. As the damage to mtDNA increases, the mitochondria become less efficient at producing energy, contributing to the aging process. In fact, mitochondrial DNA damage is so closely linked to aging that scientists can almost predict the point at which a person’s mitochondria will no longer be able to support life, leading to death from old age.
Accelerating Mitochondrial Decline
Various factors can accelerate mitochondrial decline, including environmental stressors and chronic diseases. For example, individuals with chronic conditions like multiple sclerosis may experience faster mitochondrial degradation due to the body’s constant state of stress. Additionally, lifestyle factors such as smoking and excessive alcohol consumption can further exacerbate mitochondrial damage, leading to earlier onset of age-related diseases.
The Concept of Mitochondrial Bioreactors
The Idea Behind Mitochondrial Bioreactors
Given the crucial role of mitochondria in aging and disease, researchers are exploring innovative ways to combat mitochondrial decline. One such approach is the concept of mitochondrial bioreactors. The idea is to periodically boost an individual’s mitochondria by introducing new, healthy mitochondria grown externally. This could potentially prevent or even reverse age-related diseases by replenishing the body’s supply of functional mitochondria.
How Mitochondrial Transplantation Works
Mitochondrial transplantation is an emerging field that has gained attention in recent years. The process involves extracting healthy mitochondria, typically from younger individuals or from specially cultured cells, and introducing them into the patient’s body. This process is somewhat analogous to a blood transfusion, but with the specific aim of restoring mitochondrial function.
Interestingly, mitochondrial transfer is not entirely foreign to the body. It naturally occurs as mitochondria are constantly being shifted around the body via the bloodstream and within organs like the brain and heart. This pervasive and evolutionarily conserved process highlights the potential for therapeutic mitochondrial transplantation.
Applications of Mitochondrial Bioreactors in Medicine
The potential applications of mitochondrial bioreactors in medicine are vast. For instance, they could be used to treat conditions like heart disease, where the heart’s energy demands exceed the supply provided by aging mitochondria. By introducing new mitochondria into the heart tissue, it’s possible to rejuvenate the cells and improve heart function. Similarly, mitochondrial bioreactors could be used to treat neurodegenerative diseases like Alzheimer’s by restoring energy production in brain cells.
The Science Behind Mitochondrial Transplantation
Mitochondrial Transplantation: A New Frontier
Mitochondrial transplantation is a groundbreaking technique that has been shown to be effective in various experimental models. For example, in studies conducted on mice, researchers have successfully used mitochondrial transplants to reverse the effects of sepsis, a life-threatening condition caused by a severe infection. By injecting young, healthy mitochondria into older mice with sepsis, the researchers were able to significantly improve the mice’s survival rates, demonstrating the potential of this technique to restore health in aging or diseased tissues.
Mitochondrial Transfer in Humans
While still in its early stages, mitochondrial transplantation has already begun to be used in human medicine. For instance, it has been employed in heart surgery and in the treatment of rare childhood diseases. The process involves extracting mitochondria from the patient’s own cells or from a donor, growing them in a bioreactor, and then reintroducing them into the patient’s body. This approach offers the possibility of treating a wide range of conditions by restoring the energy production capacity of cells.
The Future of Mitochondrial Transplants
Looking ahead, the goal is to develop a global network of bioreactors capable of producing healthy mitochondria on demand. These mitochondria could then be used to treat a variety of age-related and degenerative diseases, potentially extending both healthspan and lifespan. While much work remains to be done, the progress made so far suggests that mitochondrial transplantation could become a foundational component of future medical treatments.
The Role of Mitochondria in Longevity
Mitochondrial Health and Lifespan
The decline in mitochondrial function is one of the most significant factors contributing to aging. As mitochondria become less efficient, the body’s ability to maintain homeostasis and repair damage diminishes, leading to the gradual deterioration associated with aging. However, by restoring mitochondrial function, it may be possible to not only extend lifespan but also improve healthspan, the period of life spent in good health.
Mitochondrial Transplants as a Longevity Treatment
Mitochondrial transplants could play a crucial role in future longevity treatments. By periodically replenishing the body’s supply of healthy mitochondria, it may be possible to slow or even reverse the aging process. This approach could help individuals maintain their physical and cognitive abilities well into old age, allowing them to enjoy a higher quality of life for longer.
The Future of Mitochondrial Research
As research into mitochondrial function and transplantation continues, we are likely to see significant advancements in the treatment of age-related diseases and the extension of healthy lifespan. The development of mitochondrial bioreactors and the refinement of transplantation techniques will be key to unlocking the full potential of this promising field.
Conclusion: The Promise of Mitochondrial Enhancement
A New Era in Medicine
The research into mitochondria and their role in aging and disease represents a new frontier in medicine. By understanding and harnessing the power of these tiny organelles, we may be able to develop treatments that not only extend life but also improve the quality of life as we age. The potential to restore mitochondrial function through bioreactors and transplantation offers hope for addressing some of the most challenging health issues of our time.
The Path Forward
While the science of mitochondrial enhancement is still in its early stages, the progress made so far is promising. Continued research and development will be essential to bring these treatments to the broader population. As we move forward, the integration of mitochondrial therapies into mainstream medicine could revolutionize the way we approach aging and disease, ushering in a new era of health and longevity.