Since there are so many areas to research even within this arena, this page will be limited to weakly intense electromagnetic radiation that the general population is exposed to commonly. This obviously leads into topics like cellular phone use and more subtle affects of radiation to genetic material!
“The question Is not”
If cell phone radiation can cause DNA damage…
Below images prove without question it can!
“The question is”
Can your body repair DNA damage
without mutating genes?
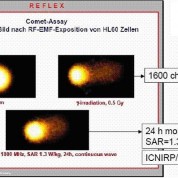
Dr. Lai and Singh found double-strand DNA breaks after RF exposure similar to Cell Phone levels.
A Comet Tail Of Your DNA From RF Exposure Below Current FCC Safe Exposure Standards

Learn how above images of DNA were taken (Click Here)
Human DNA and chromosome breakage:
Implications for cancer and neural damage!Recent US studies are showing more significant bio-effects at lower and lower power densities. (See Above) Dr. Henry Lai has reported DNA single and double strand breaks at levels below the current FCC exposure standard. Magras & Xenos have reported irreversible sterility in mice after 5 generations of exposure to .168 to 1.053 microwatts per square centimeter in an “antenna park.” Note that the current, applicable US exposure standard would be 579 microwatts per square centimeter, — 500 times higher! — and that this very low exposure level would relate more to a person living near a cellular tower, than a cell phone user.
The DNA strands form a spiral-staircase-like helix, and so breaks on only one side of the ladder are much easier to repair than those where both sides are broken. But in later experiments Lai and Singh found double-strand DNA breaks after similar exposures times and levels.
It is possible for the cell to make mistakes when repairing single-strand breaks, but the likelihood of serious mistakes (mutations) increases substantially with double-strand breaks.
Another aspect of the Lai-Singh research (with pulsed microwave similar to GSM cell phones and radar) was also disturbing. Rat brains which were excised and prepared quickly for the assay showed fewer breaks, while those which were checked four hours after exposure revealed much higher levels. This suggests that both the damage and the repair-initiation are not simple and immediate processes, and supports the thesis that DNA damage from repeated uses of a cellphone could be cumulative.
DNA And The Microwave Effect
Penn State University
January 20, 2001Can microwaves disrupt the covalent bonds of DNA? The fundamentals of thermodynamics and physics indicate this is impossible. Numerous studies have concluded that there is no evidence to support the existence of the ‘Microwave Effect’, and yet, some recent studies have demonstrated that microwaves are capable of breaking the covalent bonds of DNA. The exact nature of this phenomenon is not well understood, and no theory currently exists to explain it. This report summarizes the history of the controversy surrounding the microwave effect, and the latest research results.
(Click Here)
The Lorentz force
(researched at the cellular level, surmised at the genetic level)The major contributing factor to the ‘microwave effect‘ is actually a reciprocating lorentz-force (force exerted on a charge-carrying substance in the presence of mutually perpendicular electric and magnetic fields – such as in a microwave) exerted on the uneven charge distribution of the DNA/RNA molecule. Thus providing a non-thermal explanation for this phenomenon. If that is the case, then the frequencies involved would almost certainly be very different to the conventional 2450 MHz, since the structures and the forces involved are so different. It would become a microscopic structural resonance issue, as opposed to a purely thermal or purely chemical effect. This would also explain the similarities between the microwave effect and the external-heating method.
With the limitations proposed, there are two major ways genetic damage can occur. You can damage genetic material with temperature (fry the DNA), or you can blame the damage on physical forces (smash the bonds of the DNA causing it to misread RNA).
Northwestern University Physics & Astronomy Department – Phyx 135-2 (General Physics) — Professor Donald Ellis (Student Projects)
Ideas proposed for things not temperature related
(throughout research):Brownian Motion: At a very general level, the phenomenon of Brownian Motion and its research basically describes how things randomly bump into each other. In this case, Brownian Motion actually results due to the thermal energy of the particle itself. The thermal energy of the Brownian Motion and its movements is actually greater by a factor of 10 than the theorized movements caused by the electromagnetic radiation. In brownian motion, if one were to analyze the a given area a, and play a movie of it at speed s, at 4a, you can get the same type of movie at speed 2s, and in general, this exponential (2 as the base) holds.
Attraction of Cells: Schwan and Aldair proposed that cells in the presence of an electric field distribute ions across the membranes so they become polarized (and therefore attracted to each other). Although this is not directly genetic damage, many cells rely on proper transport of nutrients across the membrane to be able to successfuly duplicate genetic material. This may inhibit the very delicate process of duplication.
Lorentz Effect: This is the one I will spend some more time going into detail on. Some of the literature on the material is kind of , but it gives a general idea (in addition, I could find more on this topic than I could on the other two). Because I have kind of a push on this effect and the fact that it relates very well to course material, this topic will be explored more.
The literature concentrates on cellular damage due to the Lorentz Force, but it also uses this damage as a potential gap-filler for the Microwave Effect.
Let’s suppose, for a second, that there is some damage to the hydrogen bonds (this damage would also translate to the covalent bond, but to about 10 times less a degree). The force due to direct EMR:
FB = qv X B
The energy of this force is the sum of all FB projected over a certain distance (dot product). For simplicity, lets assume that the force acts along a straight line over the length of the hydrogen bond. The magnetic field will also be I know that there are a lot of cellular dynamics left out here, but the calculations would get fearfully complex otherwise.
Requirements for this force to matter:
– the ion(s) must be moving near the DNA
– they must be moving at a velocity in which the resulting energy is a visible fraction of the total energy to break a hydrogen bond
– we are assuming worst-case scenario where all conditions dependent on time are maximizedQuestion: What would be the required frequency to produce x percentage of the energy required to break the H-Bond?
Using these equations (some simplified from assumptions)
FB = qv X B ==> FB = qvB (magnetic field is perpendicular to velocity)
U = Integral(Dot( FB, s) ) ==> U = FBs (where s is the distance across the hydrogen bond)
E/B = cE = -grad(V) = -uodqow2/4pi ( sin (z)/r ) cos(w(t-r/c) ) z ====> E = kw2/r (assume head-on radiation at maximum amplitude) note that r is the distance from the EMR source.
Doing these processes and some algebra, we get:
find B from E
substitute in F
plug in U
solve for w
w = k Ubreak x r /s where k = uodq2/4pi (d and q are related to the dipole moment from the antenna)This makes sense with intuition. The frequency required to achieve x percentage (ie .75) breakage of the H-Bond (Ubreak) is directly proportional to the bond energy, the distance the radiation source is from the DNA, and inversely proportional to the size of the bond needed to be broken. Assuming the radiation is 10cm away from the nearest DNA strand in a brain cell of a child and that the H-Bond length is about 2 Angstroms which is very roughly 10-10 m. k is about 3 x 104. These are all of course rough values. The k is based off of the the energy of the microwave given in the Temperature section above (plug into equation and find constant).
Percentage of H-Bond
Frequency
10%
1.5 x 1012Hz
50%
7.5x 1012Hz 100% 1.5 x 1013Hz So in any case, the general point is made. You need frequencies much greater (as expected) than a typical microwave to break the bonds of DNA solely with the Lorentz force, but it probably has some effect.
Continue this student research on RFR and DNA damage
Radiation can produce a break in a strand by destroying a P-E bond. Think of radiation as blasting away a electromagnetic bonds in one side of the ladder. We will call such damage a single-strand break (SSB). While relatively weak, the electromagnetic hydrogen bonds between nucleotides cannot be permanently broken by such a radiation hit. An isolated SSB also does no permanent damage to the molecule; it is soon repaired.
The effect of the radiation may not be to kill the cell, but to alter its DNA code in a way that leaves the cell alive but with an error in the DNA blueprint. The effect of this mutation will depend on the nature of the error and when it is read. Since this is a random process, such effects are now called stochastic. Two important stochastic effects of radiation are cancer, which results from mutations in nongerm cells (termed somatic cells), and heritable changes, which result from mutations in germ cells (eggs and sperm) BIRTH DEFECTS.